PHYSICIAN'S GUIDE
TO
PESTICIDE POISONING
Table of Contents | Preface | Section I | Section II | Section III | References
WHERE TO GET IMPORTANT INFORMATION
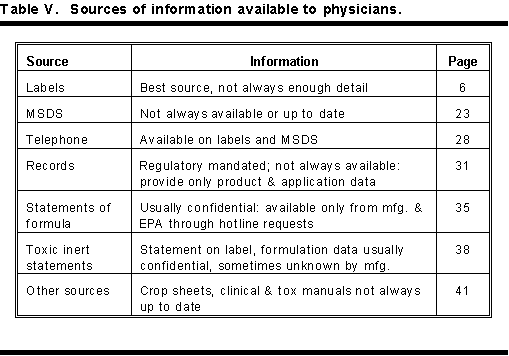
There are six basic sources that give fast information to physicians on recognizing and treating pesticide poisoning. The most important of these provide information on the nature of the toxic agent, the specific cause of the poisoning, and how to treat poisoning from these compounds. Table V lists these sources, availability and the page in this handbook that gives amplified information on these information sources.
In the order of importance and availability, these sources are labels, material safety data sheets, telephone sources, application records, statements of formula, toxic inert ingredient statements, crop sheets, and manuals on clinical treatment and toxicology of pesticides.
A pesticide label is the most important single source of information a physician can have when treating pesticide poisoning. Pesticide labels are legal documents. EPA and the State Department of Agriculture, must approve labels before a pesticide enters the market. Figure 1

Labeling is all the information the manufacturer provides on a pesticide product. Labeling includes the label on the product container and package. It also includes brochures, leaflets, bulletins, and manuals, and any separate information available from pesticide dealers or a recognized authority.
Figure 1 shows an example of the front panel of a category I, restricted-use pesticide. Note the standard format and the various parts of the label designed to provide the physician with information.
To physicians, the label is a source of information on proper treatment for poisoning cases. Labels are NOT all the same. Remember to read the label of each product.
In recent years, EPA has adopted a standard format for pesticide labels. However, all the information a physician needs is not in one place. Instead it is scattered in several places throughout the pesticide label. Knowing where to look on the standard format of a pesticide label enables a physicians to quickly find urgently needed information. Almost all of the information of value to a physician is on the front panel of a pesticide label.
Table VI contains a list of all the various parts of a pesticide label. The parts that are important to a physician are in bold letters.
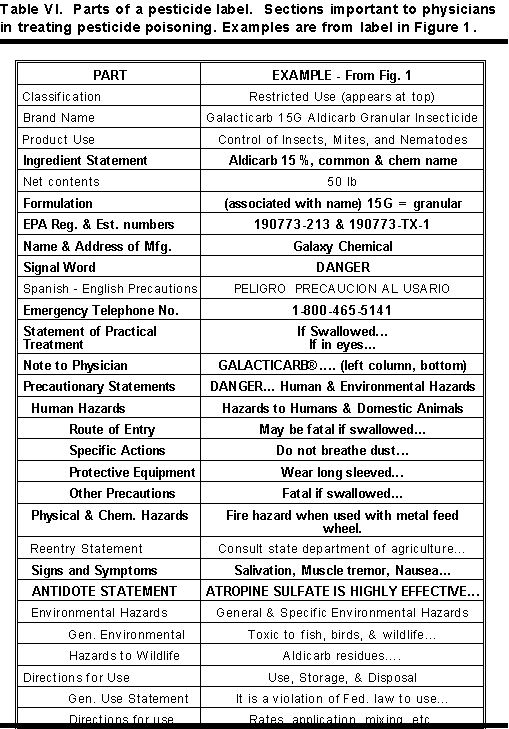
Table VII shows a condensed list of the parts of a pesticide label important to a physician. The less important parts in treating pesticide poisoning have been omitted. To see the relative positions of this information on the label, see Figure 1 and Table VI.
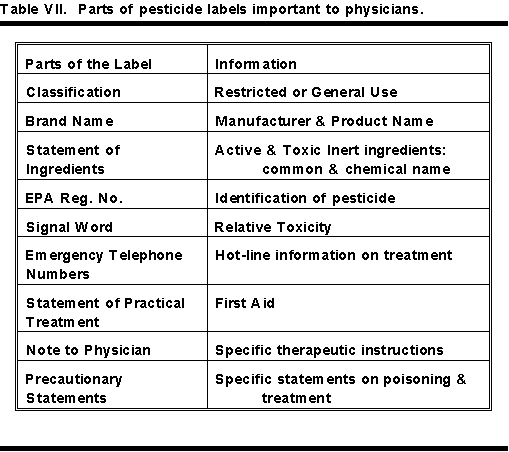
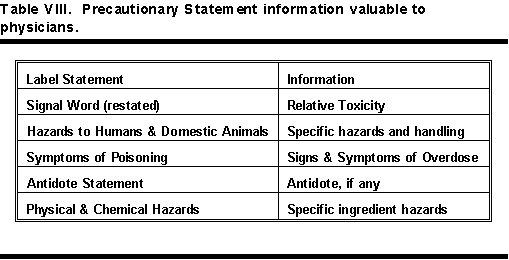
There is much valuable information in the precautionary statement part of a label. This is particularly true for products with the signal word DANGER on the label. Table VIII shows information valuable to a physician available in the precautionary statements.
There are other parts to pesticide labels, and physicians may have to sort out information on older pesticide labels. The simple fact that a person shows up in an emergency room with poisoning symptoms and a pesticide label, does not mean that the pesticide meets present EPA standards. The product may have been packaged many years earlier, before present EPA requirements took effect. Many homeowners keep pesticides on shelves for years and even decades. Many of the old labels may not have all the information needed. It is important to know where to look for information.
On older pesticide labels, the front panel will have a minimum of the brand name, active ingredients (no toxic inerts), and signal word. It may have some of the precautionary statements, signs of poisoning, and notes to physician. However this is not a sure thing.
There are many facts to learn from the Label. These include Chemical hazards, registered uses, recommended rates, compatibility, and phytotoxicity. Information on the label generally pertains to either product identification or proper product use. Below are some details about specific statements on pesticide labels.
Pesticide labels are divided into several parts. There are various statements and blocks of information that must be on every pesticide label. Highly toxic pesticides require additional information on labels. Some labels require special warnings and precautionary statement. See Table VI for the parts of a label and examples.
Brand Name: BEWARE OF BRAND NAMES!
Table IX shows nearly identical brand names with very different active ingredients. Table X shows pesticides with very different brand names having identical active ingredients.
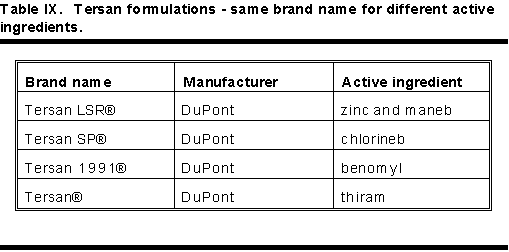
Physicians must beware of brand names. Always use active ingredient statements on the label to determine the active ingredient.
Physicians must not simply rely on the
memory of poisoning victims or co-workers who may give only the brand name
of a pesticide product. The product label is the only way to find out what
the active ingredients are.
Classification
EPA classifies every use of every pesticide as either "general use" or "restricted." Restricted-use pesticides carry the statement at the top of the label in Figure 1. As the message at the top of the label implies, restricted-use pesticides require licensing for purchase and use.
Net Contents and ingredient statement
The front panel of the label shows the net contents that is, how much product is in the container. Each label also must list what is in the product. the list shows active and inert ingredients. It also shows the amount of each.
Active ingredients are the chemicals that control the target pest. they must be identified by their chemical name or official common name. Most products also have inert (inactive) ingredients. These do not act on the target pest. However, they may contribute to poisoning (See Toxic Inerts later in this chapter).
Except in the case of biological and botanical pesticides, the ingredient
statement lists chemical names (See Figure 1). Many chemical names are
called by a shorter common name. Common names may be used in the
ingredient statement only if they are accepted by EPA. Figure 1 shows examples
of common and chemicals names.
Type of pesticide and formulation
The type of pesticide usually is listed on front of the label. This short statement tells what kind of pests the product control. Figure 1 shows an example of a soil insecticide in a granular formulation. There are many formulations.
The more common formulations have accepted abbreviations. Table XI shows some of the abbreviations for common pesticide formulations.
Registration and Establishment numbers
These numbers identify specific pesticide formulations and where they come from. The registration number identifies a specific formulation with a specific set of inert ingredients. Establishment numbers identify where a pesticide was made. These numbers are necessary in case of accidental or deliberate poisoning. These numbers also are necessary in the case of claims of misuse, faulty products or liability.
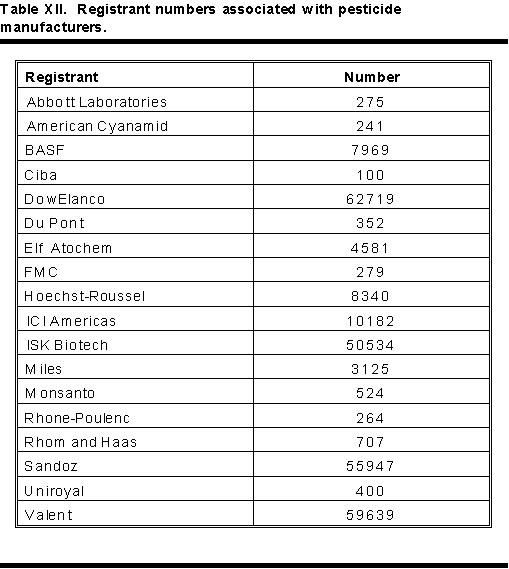
An EPA registration number appears on all pesticide labels. Most products contain only two sets of numbers. The example in Figure 1 shows EPA REG. no. 190773-213. The first set of digits, 190773, identifies the manufacturer. The second set, 213, identifies the product. Examples of a few manufacturer or registrant numbers appear in Table XII. Each time a pesticide changes hands, the manufacturer number changes to follow it. Frequently, the product number changes also. Physicians must be aware of possible changes in registration numbers.
The establishment number, EPA Est. No. 190773-TX-1 appears on
either the label or container. The number identifies the facility that
produced or formulated the product. Pesticide labels must also have the
name and address of the manufacturer. A maker or distributer of a product
must list its full company name and address (See Figure 1). Physicians
can use the name and address on the label to contact pesticide manufacturers
in case of emergencies.
Product Use
The remaining parts of the label pertain to proper product use rather than product identification. These important parts of the label include signal words and symbols, precautionary statements, storage and disposal instructions and directions for use (See examples in Table VI).
Signal Word
Every label has a signal word required by the EPA. These are "DANGER," "WARNING" "CAUTION." This word gives a signal of how dangerous the product is to humans. The signal word does not tell the risk of delayed effects or allergic reactions. The signal word appears in large letters on front of the label usually next to the statement, "Keep Out of Reach of Children," which is required on every product.
DANGER. This word signals that the pesticide is highly toxic, or could cause severe eye or skin injury. Highly toxic pesticides also carry the skull and crossbones symbol and the word POISON printed in red. Pesticides than can badly damage the skin or eyes may have the signal word DANGER without the word POISON.
WARNING signals any product that is moderately toxic.
CAUTION signals any product that is slightly toxic.
Table XIII - Route of entry

This notice follows the signal word and tells which route of entry (mouth, skin, eyes, lungs) needs special protection (See Figure 1 and Table XIII).
Specific actions
In addition to route of entry, the label may list specific actions needed to prevent poisoning accidents. These include things to avoid and the kind of protective equipment to wear. Figure 1 shows several, including: "Do not breathe dust.
Protective clothing and equipment
Some labels fully describe protective equipment you may need to handle contaminated victims. Many labels have no such message.

Other precautions
Labels often list other precautions to take. Always take the actions in Table XIV, whether or not they are stated on the label.
First Aid, Note to Physician and Statement of practical treatment
These statements tell physicians and emergency personnel what to do in case of pesticide poisoning. See the Statement of Practical Treatment and the Note to Physician in the example in Figure 1.

All DANGER labels must include a section of First Aid Treatment, Poison Signs or Symptoms, Note to Physicians (or Antidote), and an Emergency Assistance Call telephone number. WARNING and CAUTION labels may have only an Emergency Assistance Call telephone number. Table XV lists statements on DANGER labels that are valuable aids to physicians and other health care professionals. These are not all in the same place. See Table VI for relative positions of each of these statements.
Physicians should advise patients with suspected pesticide poisoning to bring the pesticide label with them. This is very important. The pesticide label can provide the physician will most of the information necessary to save the life of someone suffering from pesticide poisoning. Without the label, treatment of many pesticide poisonings will be like shooting in the dark.
OTHER PARTS OF A LABEL
Hazards to wildlife and the environment
Some products are classified RESTRICTED USE because of environmental hazards alone.
General environmental statements
These statements appear on nearly every pesticide label.
Physical and chemical hazards
The physical and chemical hazard section of the label warns pesticide users of any fire, explosion or chemical hazards. See examples in Figure 1 and Table VI.
Reentry statement
Some pesticide labels contain a reentry precaution. This tells the applicator how much time must pass before people can reenter a treated area without protective clothing. Figure 1 and Table VI give an example of the reentry statement.
Storage and disposal
All labels give general instructions for proper storage and disposal. Table VI and Figure 1 show examples of storage and disposal statements.
Table XVI Directions for use

This section of the label gives specific information on how to use the product. Table XVI lists some of the information available in the directions for use part of the label. This is the part of the label that provides information for proper use. Any use other than that listed on the label is misuse. This is an important source of accidental poisoning. Physicians may find keys to poisoning and how it occurred by looking in the directions for use part of the label.
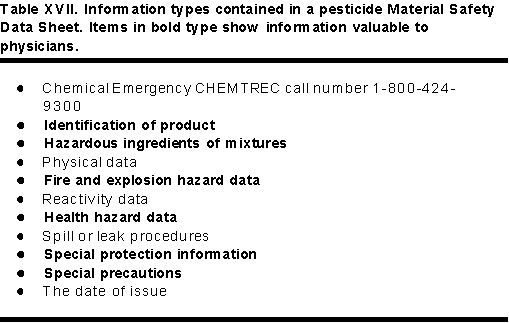
The Occupational Safety and Health Act (OSHA) required businesses that store and use chemicals to maintain several records. One of these is a workplace chemical list. The other is a file of Material Safety Data Sheets (MSDS). A MSDS is a document that lists various characteristics of a chemical that may contribute to its safety in storage, transportation, use or disposal. A MSDS has 10 sections. Table XVII displays the types of information in the various sections of a MSDS. Displayed in bold letters are the parts of the MSDS that physicians will find helpful in the diagnosis and treatment of pesticide poisoning.
Hazard Communication Standard
The Hazard Communication Standard is a rule written and enforced by OSHA. It protects employees who may be exposed to hazardous chemicals under normal operating conditions. Chemicals in HCS include pesticides. The terms exposure and exposed refer to the contact of an employee with a hazardous chemical in the course of employment.
Superfund Amendments and Reauthorization Act (SARA Title III)
SARA Title III is known by some as "The Community Right-to-Know Act". It is a federal right-to-know law that affects those who produce or store hazardous chemicals. Section 311 includes the reporting of material safety data sheets. It is one physicians should know about. Under Section 311, Employers must obtain and keep material safety data sheets (MSDS) and submit copies of each sheet or a listing to their local fire department, the LEPC and the SERC. Household and agricultural chemicals are excluded from this rule.
Section I Identification of Product
This section includes the manufacturer's name and address, the trade name and synonyms for the product, the chemical name and synonyms, the chemical family, and emergency telephone numbers.
Section II Hazardous Ingredients of Mixtures
This section is of value to medical professionals who may have to treat pesticide poisoning. This section shows components of the pesticide, active ingredients, some inert ingredients, threshold limit values.
Section III Physical Data
The physical data section has information that includes appearance and odor, and chemical constants.
Section IV Fire and Explosion Hazard Data
This section is of value to some emergency response personnel, including fire departments, medical, and police. It includes data and unusual fire and explosion hazards.
Section V Reactivity Data
This section contains information on stability, incompatible materials (materials to avoid), hazardous decomposition products. Emergency response and medical personnel may find useful information in this section.
Section VI Health Hazard Data
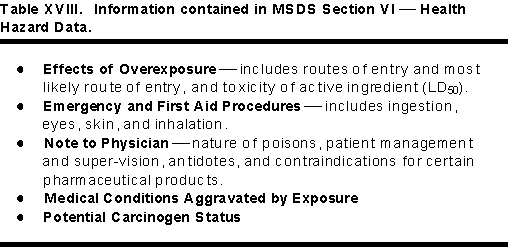
This section is very valuable to medical personnel and health care professionals dealing with chemical emergencies or suspected pesticide poisoning. The Table XVIII lists the important information in the Health Hazard Data section of a MSDS. It includes effects of overexposure, emergency and first aid, note to physician, medical conditions aggravated by exposure, and potential carcinogen status.
Section VII Spill or Leak Procedures
This section includes actions in case material is released or spilled.
Section VIII Special Protection Information
This section may prove valuable to health care professionals if they are attempting to determine causes and effects of a pesticide exposure or dealing with contaminated patients.
Section IX Special Precautions
This section lists special handling, storing and other precautions.
Section X Date
This section shows the date of issue and any previous MSDS that the
present one supersedes.
Table XIX - TELEPHONE SOURCES: HOTLINES, EMERGENCY NUMBERS, ETC.
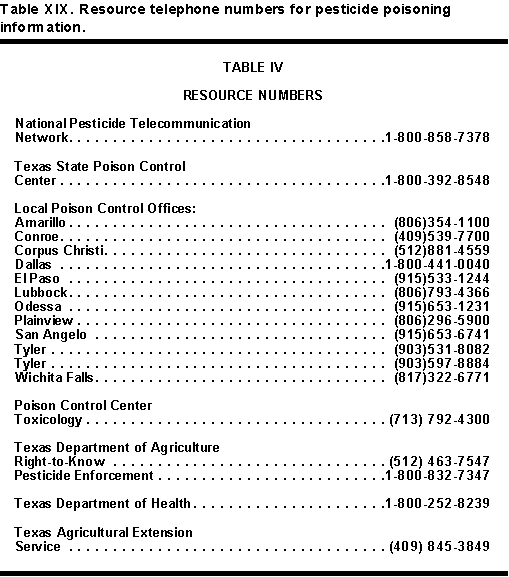
There are many places to call, if physicians and health care personnel only know the numbers. There are national, state, and regional poison control centers. There are company telephone hotlines for chemical emergencies. Poison control centers have general clinical information readily available. Company hotlines can inform the physician about special considerations in their products. These company hotlines are the only way to get information about possible toxic effects of inert ingredients. Table XIX lists some common resource telephone numbers general clinical or pesticide information. These include the National Pesticide Telecommunications Network (NPTN), poison control centers, and government agencies.
Another important telephone source of information exists in pesticide manufacturer emergency hot-lines. Many pesticide labels and most material safety data sheets have these telephone numbers. Table XX lists a few chemical company information numbers and hotlines taken from Material Safety Data Sheets (MSDS) for their products.
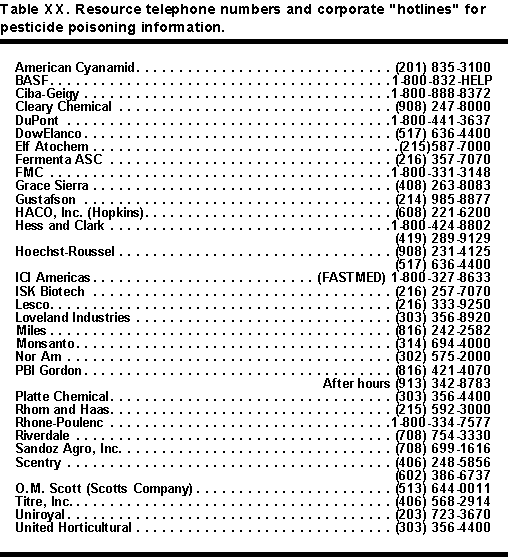
Company hotlines can provide specific information about pesticide formulations and specific hazards and treatment. DANGER labels must all bear a special emergency "hotline" telephone number. Physicians should not hesitate to call company emergency hotlines for information on pesticides. These hotline numbers are on all pesticide labels that bear DANGER as the signal word. They also are on all material safety data sheets (MSDS). Emergency telephone numbers in Table XIX and the company "hotlines" in Table XX may prove very valuable in treating pesticide poisoning.
Company hotlines can inform the physician about special considerations in their products. These company hotlines are the only way to get information about possible toxic effects of inert ingredients.
PESTICIDE RECORDS RECORDS PESTICIDE USERS MUST KEEP
When dealing with suspected pesticide poisoning or exposure, it is vitally important for physicians and health care providers to know what pesticides have been applied. If labels and MSDS are not available, there are certain records required by law that will also have valuable information. The law also requires those who keep the records to provide them to physicians on demand. This information is available from pesticide application, storage, and transportation records. Many laws require the keeping of these records. These records can be very valuable, if the physician knows that it exists and knows how to get it. Table XXI shows some of the records available and laws governing them.
Pesticide users, haulers, applicators, distributors, retailers and manufacturers are required by law to provide records to physicians and other emergency personnel. When a medical or other emergency involving pesticides is suspected, physicians may demand information contained in pesticide records.
Physicians and health care professionals may request pesticide records required by these laws to assist in diagnosis and treatment of suspected pesticide poisoning.
It is important for physicians to know that they have a right to
this information under law. A physician may demand pesticide records in
order to treat persons with suspected pesticide injuries.
Table XXI. Pesticide Records Mandated by Law*
| 1990 Federal Farm Bill and 7 CFR 110 | Federal Worker Protection Standard 40 CFR 170 | Texas Agricultural Hazard Communication Law & Regs | Texas Pesticide Law and Regs | Texas Herbicide Law and Regs |
| Private Applicators | Producers of Agricultural Plants using pesticides | Covered employers of farm workers*** | Commercial and noncommercial applicators | All applicators using regulated herbicides in regulated counties |
| Restricted-use pesticides | All pesticides | All pesticides*** | All pesticides | Regulated herbicides |
| Month, day, and year of application | Before application post list that includes application time & date | Work Place Chemical List one form for each crop, work area, or workplace with: Date of application, use, or storage | Date of application and times of day including each time application starts and ends | Date of application and the time of day |
| Person for whom the application was made | Person for whom the application was made | |||
| Application location; the site treated (name of crop, etc.); and total acres treated | Location and description of area to be treated | Location of treated area and/or storage; name of crop(s); (acres treated required on form) | Location of the application; the site treated (name of crop, etc.); and total acres treated | Location of the application; the site treated (name of crop, etc.); and total acres treated |
| Brand name or product name and EPA Reg. No. | Product name; EPA Reg. No.; and active ingredient | Product name (applied or stored) and EPA Reg. No. | The pesticide applied including product name; EPA Reg. No.; active ingredient(s); spray diluents and surfactants | The pesticide applied including product name and EPA Reg. No. |
| Restricted Entry Interval (REI) | ||||
| (also, label(s) must be available to handlers during any pesticide handling task | ||||
| estimated amount of product per acre or stored required on form | Rate of active ingredient per unit | Rate of product per unit | ||
| Total amount of restricted-use pesticide used | Total amount of active ingredients and total volume of materials applied per unit | Total volume of materials applied per unit | ||
| Material Safety Data Sheet(s)
(MSDS) (on file for each pesticide) |
||||
| Name of the pest treated | Name of the pest treated | |||
| Current crop sheets (on file for each crop) | ||||
| Climatological data including wind direction and velocity, air temperature, etc. | Climatological data including wind direction and velocity, air temperature, etc. | |||
| Name of certified or licensed applicator making or supervising application | Applicator name and license no. | |||
| FAA "N" number of aerial equipment, or decal number affixed to the unit | FAA "N" number of aerial equipment, or decal number affixed to the unit | |||
| ***For each pesticide or hazardous fertilizer or other hazardous chemical with 500 lbs or 55 gallons stored or used | ||||
| ** Commercial applicators must provide records of application within 30 days to the person for whom the application was made. | ||||
| Maintain records for 2 years | Display for 30 days after reentry interval (REI) has expired | Maintain records for 30 years; may transfer to TDA annually | Maintain for 2 years | Maintain for 2 years |
*Consult appropriate laws and regulations for full details. Also, special record keeping requirements for Compound 1080 Livestock Protection Collars and M-44 cyanide capsules are in section 7.32 and 7.33 respectively of the Texas Pesticide Regulations and in the product labeling.
Pesticide formulations consist of a blend of ingredients. On the label, the active ingredients are usually the only ones listed. The rest of the ingredients are inert ingredients. These are listed for registration purposes in the confidential statement of formula. Physicians or the general public seldom, if ever, are made aware of inert ingredients in pesticides. Only the active ingredients are listed in packaging and on MSDS sheets. An exception is in the EPA policy on toxic inert ingredients.
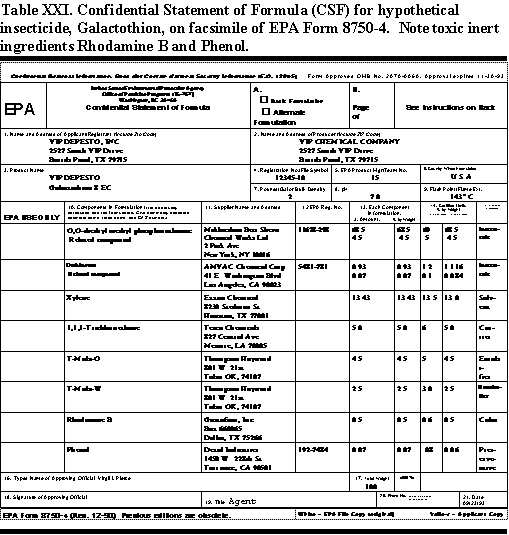
In the Confidential Statement of Formula, EPA requires the registrant to list all ingredients that go into a pesticide formulation. These ingredients include all active and inert ingredients, the weight per batch, the percentage in the formulation, and sources of the ingredients. Table XXII shows a facsimile of EPA Form 8750-4 with a Confidential Statement of Formula.
The CSF is exactly what it says confidential. Only the registrant and the EPA knows what is in it. It is not available to the public, medical professionals, or emergency personnel. It takes special emergency requests for information, primarily to the registrant to get medical information on the ingredients in a pesticide. CSF information on formulations that may contribute to poisoning or create a health hazard in those exposed to a pesticide is not easy to get. Sometimes, it is impossible to get, particularly when formulators use inert ingredients from suppliers who do not divulge their contents and regard them as trade secrets.
Know how to use pesticide labels
It is extremely important for physicians to know how to use the label on pesticide products to get information from manufacturers. All DANGER labels will have emergency "hot line" numbers for medical personnel to call. This is the only way to get information on medical treatment for chemicals not listed in the active ingredients. It is the ONLY way to get information on toxic inert ingredients.
Pesticide active ingredients are not the only part of a pesticide formulation that may pose health hazards. Until 1987, unstated ingredients on the label also posed significant health hazards to poisoning victims. On april 22, 1987, regulations published in the Federal Register required pesticide registrants to add warnings.
EPA developed several lists of inert ingredients included in pesticide formulations that required specific warnings. We include toxic inerts on EPA List 1 and List 2. These are in Tables XXIII and XXIV. List 1 contains toxic inert ingredients with known toxicities. List 2 contains toxic inert ingredients with undetermined toxicity and a high priority for testing. Only ingredients on List 1 required the following warning on the front panel of the label. The warning will be near the ingredient statement in a type size comparable to that found in other front panel statements
.
Table XXII shows the inerts of toxicological concern in List 1.

Because of concern that some inert ingredients in pesticide products might cause adverse effects to humans or the environment, EPA developed a strategy for the regulation of inert ingredients.
Table XXIII shows the inerts of toxicological concern in List 1.

Most of the chemicals on List 2 have been designated for testing through several government agencies. EPA has been reluctant to register any formulation with inert ingredients on list 2. However, to date, no "toxic inert warning statement" has been required for inert ingredients on List 2.
OTHER SOURCES OF INFORMATION ON PESTICIDE POISONING
Other valuable sources of information exist on pesticide exposure and treatment. Some of these are government publications such as Texas Department of Agriculture Crop Sheets and clinical manuals on toxicology and treatment of pesticide poisoning.
The Texas Right-to-Know law also has another valuable item of information. This is the Crop Sheet. Agricultural employers who qualify under the Texas Agricultural Hazard Communication Act must provide workers with pesticide safety training and give them crop sheets for the crops they work in.
The crop sheet is printed in Spanish and English. It gives certain general information about pesticides used in particular crops in particular regions of Texas. The crop sheet also has symptoms of poisoning. The crop sheet also has specific information on it. It lists many specific pesticides and restricted entry intervals for particular crops in specific regions of Texas. Crop sheets also list poison control center hotline telephone numbers. The crop sheet can provide valuable information to physicians treating agricultural workers.
CLINICAL MANUALS AND TOXICOLOGY GUIDES
Among the valuable sources of information on toxicology and treatment are Hayes' (1991) three volume set on pesticide toxicology and EPA's publication of Daniel Morgan's excellent manual. Another excellent source, although somewhat out of date, is Gosselin et al. (1984) Clinical Toxicology of Commercial Products. Table XXV lists some reference sources on diagnosis and treatment of pesticide poisoning.
SUMMARY OF INFORMATION SOURCES
There are many sources of pesticide information available to physicians and other health care professionals. Most important is to know where to look. Among the sources of information available to the physician are labels, formulation data, and material safety data sheets. Other sources of information include telephone numbers of poison control centers, crop sheets provided under the Texas Agricultural Hazard Communication Act (Agricultural Right-to-Know), pesticide application records, and workplace chemical lists. Other sources of valuable information are clinical guides like Morgan (1989) and toxicology handbooks like Hayes and Laws (1991). Table V summarizes the sources of information available to physicians.
Table of Contents | Preface | Section I | Section II | Section III | References